
But in the case of Pi bonds, there is no such symmetry exists that is why they are unsymmetric.A covalent bond is formed by lateral or sideway or collateral overlapping of pure orbitals is known as a pi bond. There is a cylindrical charge symmetry that exists between the Sigma bond’s axis. When two atoms interact the bond which is formed first is a sigma bond and then only a pi bond starts forming. Sigma bonds will determine the shape of the molecule while pi bonds have no role in the case of shape. Those atoms with the Sigma bonds are highly reactive while atoms with pi bonds are less reactive.
#Sigma bond example free
But pi bonds can be accessed only with the help of a sigma bond and free rotation of molecules is not possible. Sigma bonds can exist independently and free rotation is possible in the case of Sigma bonds. But in the case of pi bond overlapping orbitals are only pure orbitals. In the case of sigma bonds, the overlapping of orbitals happens by pure orbitals, hybrid orbitals and one hybrid orbital, and one pure orbital. They are both covalent bonds and are used to describe some features of covalent bonds but they possess some differences they are

Sigma bonds and pi bonds are types of bond formed in covalent bonds. NCERT Exemplar Solutions for All Subjects.NCERT Exemplar Class 12th Chemistry Solutions.NCERT Exemplar Class 11th Chemistry Solutions.And for an antibonding molecular orbital the probability of finding an electron is low and correspondingly the electron density is low. While the antibonding molecular orbital is formed by the combination of – and + part of atomic orbitals and thereby no bond formation is taking place. The bonding molecular orbital is formed by the combination of + and + part or – and – part of atomic orbitals and thereby forming a bond in between the two atoms. The figure below shows the bonding and antibonding molecular orbital formation in the hydrogen molecule. And the energy difference between two bonding molecular orbital and antibonding molecular orbital is high. Only the bonding molecular orbitals participate in the bond formation. Antibonding orbitals have more energy compared to the bonding molecular orbitals. Thus thereby avoiding the extra electron interacting with other electrons. When there is a presence of extra electron like in the case of H - 2 the extra electron will be present in the antibonding orbital. The Following Figure Shows the Formation of Antibonding Orbitals. The electron cloud in the case of the pi bond is unsymmetrical and in the case of the sigma bond is symmetrical. Similarly, in the case of a triple bond, there is one sigma bond and 2 Pi bonds are there eg. A double bond contains a one sigma bond and one Pi bond eg. In the case of multiple bonds like a triple and double bond, it contains pi bonds too. A single bond always contains only one sigma bond and no pi bond is there eg. In the case of a Pi bond which is formed due to the lateral overlapping of orbitals and the bond is weaker than a sigma bond. Sigma bond is usually defined for diatomic molecules and is formed by the headways overlapping between atomic orbitals. The following image shows the pi bond formation. The following images show the formation of a sigma bond between two atoms.įor a pi bond formation, the atomic orbitals are overlapped in a sideways manner. The orbital overlapping of sigma and pi bonds is different, for a sigma bond formation headways overlapping between atomic orbitals will take place. More than one pi bond can be formed between atoms.ĭistinguishing features of sigma and pi bonds: The sigma bond that can be formed between two atoms is one. Overlapping orbitals are only pure orbitals. The overlapped orbitals formed by pure orbitals, hybrid orbitals and one hybrid orbital, and one pure orbital. It will determine the shape of the molecule.ĭo not have a role in the shape of molecules. Pi bonds can be accessed only with the help of a sigma bond.
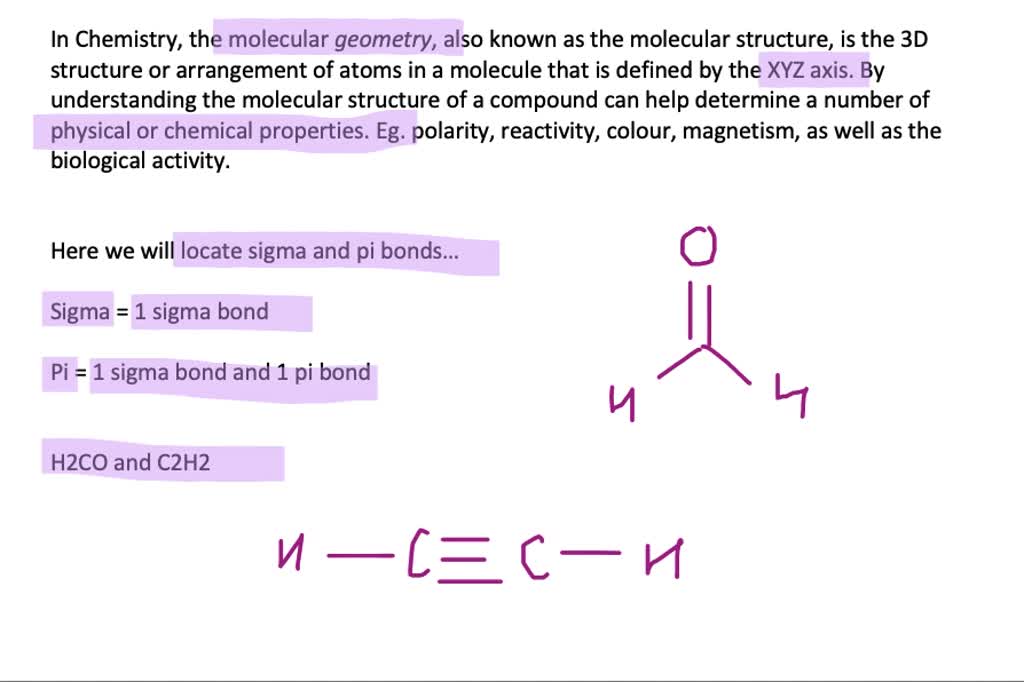
It can exist independently and free rotation is possible. Pi bonds are formed by the lateral overlapping of atomic orbitals. Sigma bonds are formed by the headways overlapping of atomic orbitals.


 0 kommentar(er)
0 kommentar(er)
